Robotics Meet X-ray Lasers In Cutting-Edge Biology Studies
Platform Brings Speed, Precision in Determining 3-D Structure of Challenging Biological Molecules
Scientists at the Department of Energy’s SLAC National Accelerator Laboratory are combining the speed and precision of robots with one of the brightest X-ray lasers on the planet for pioneering studies of proteins important to biology and drug discovery.
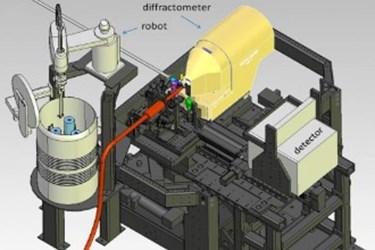
The new system uses robotics and other automated components to precisely maneuver delicate samples for study with the X-ray laser pulses at SLAC’s Linac Coherent Light Source (LCLS). This will speed efforts to map the 3-D structures of nanoscale crystallized proteins, which are important for designing targeted drugs and synthesizing natural systems and processes.
A New Way to Study Biology
"This is an efficient, highly reliable and automated way to obtain high-resolution 3-D structural information from small sizes and volumes of samples, and from samples that are too delicate to study using other X-ray sources and techniques," said Aina Cohen, who oversaw the development of the platform in collaboration with staff at LCLS and at SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL), both DOE Office of Science User Facilities.
She is co-leader of the Macromolecular Crystallography group in the Structural Molecular Biology (SMB) program at SSRL, which has used robotic sample-handling systems to run remote-controlled experiments for a decade.
The new setup at LCLS is described in the Oct. 31 edition of Proceedings of the National Academy of Sciences. It includes a modified version of a “goniometer,” a sample-handling device in use at SSRL and many other synchrotrons, as well as a custom version of an SSRL-designed software package that pinpoints the position of crystals in arrays of samples.
LCLS, with X-ray pulses a billion times brighter than more conventional sources, has already allowed scientists to explore biological samples too small or fragile to study in detail with other tools. The new system provides added flexibility in the type of samples and sample-holders that can be used in experiments.
Rather than injecting millions of tiny, randomly tumbling crystallized samples into the path of the pulses in a thin liquid stream – common in biology experiments at LCLS – the goniometer-based system places crystals one at a time into the X-ray pulses. This greatly reduces the number of crystals needed for structural studies on rare and important samples that require a more controlled approach.
Early Successes
"This system adapts common synchrotron techniques for use at LCLS, which is very important," said Henrik Lemke, staff scientist at LCLS. "There is a large community of scientists who are familiar with the goniometer technique."
The system has already been used to provide a complete picture of a protein’s structure in about 30 minutes using only five crystallized samples of an enzyme, moved one at a time into the X-rays for a sequence of atomic-scale “snapshots.”
It has also helped to determine the atomic-scale structures of an oxygen-binding protein found in muscles, and another protein that regulates heart and other muscle and organ functions.
“We have shown that this system works, and we can further automate it," Cohen said. "Our goal is to make it easy for everyone to use.”
Many biological experiments at LCLS are conducted in air-tight chambers. The new setup is designed to work in the open air and can also be used to study room-temperature samples, although most of the samples used in the system so far have been deeply chilled to preserve their structure. One goal is to speed up the system so it delivers samples and measures the resulting diffraction patterns as fast as possible, ideally as fast as LCLS delivers pulses: 120 times a second.
The goniometer setup is the latest addition to a large toolkit of systems that deliver a variety of samples to the LCLS beam, and a new experimental station called MFX that is planned at LCLS will incorporate a permanent version.
Team Effort
Developed through a collaboration of SSRL's Structural Molecular Biology program and the Stanford University School of Medicine, the LCLS goniometer system reflects increasing cooperation in the science of SSRL and LCLS, Cohen said, drawing upon key areas of expertise for SSRL and the unique capabilities of LCLS. “The combined effort of staff at both experimental facilities was key in this success,” she said.
In addition to staff at SLAC’s SSRL and LCLS and at Stanford University’s School of Medicine, researchers from SLAC’s Photon Science Directorate, the University of Pittsburgh School of Medicine, Howard Hughes Medical Institute, Montana State University, Lawrence Berkeley National Laboratory and the University of California, San Francisco also participated in this effort.
The work was supported by the Department of Energy Office of Basic Energy Sciences, the SSRL Structural Molecular Biology Program via the DOE Office of Biological and Environmental Research, and the Biomedical Technology Research Resources program at the National Institute of General Medical Sciences, National Institutes of Health.
About SLAC National Accelerator Laboratory
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Source: SLAC National Accelerator Laboratory
