Plasmid DNA Manufacturing Service

Plasmid DNA manufacturing using a unique process for Research, HQ and GMP grades
Benefits
- Purity- High purity plasmids with very low endotoxin level (< 2EU/mg)
- Robust- Unique scalable proprietary process allowing plasmid production from 50mg to 20g in Research, HQ & GMP grades
- Time-Saving- Between 4 and 7 weeks for Research Grade and 3 months for GMP grade
- Flexible- Manufacturing process compatible with all types of plasmids (from 2 to more than 20 kb, plasmids for LV, AAV, mRNA)
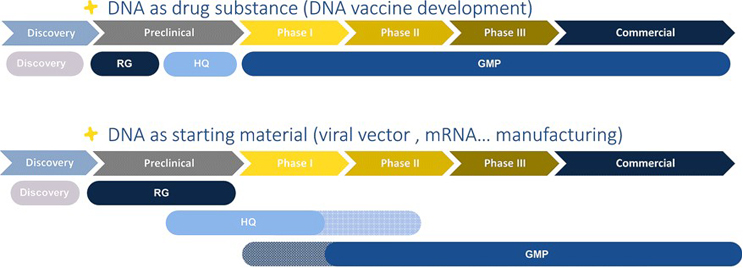
Polyplus offers a comprehensive range of plasmid services including DNA sequence design, optimization and plasmid engineering, cloning, transformation in our proprietary and IP free microbial E. coli, fermentation, patented soft DNA extraction process, single chromatography step and analytical testing.
Polyplus has developed an unique robust and scalable plasmid manufacturing process ensuring the highest plasmid purity possible with extremely low endotoxin levels (< 2EU/mg), whatever the scale and the quality grade, being research, HQ or GMP grade.
Our facility is equipped with state-of-the-art technology (stainless steel fermenters Sartorius Biostat® C+) f and is operated by a team of experienced scientists and technicians for fast fermentation development and optimization.
Capabilities
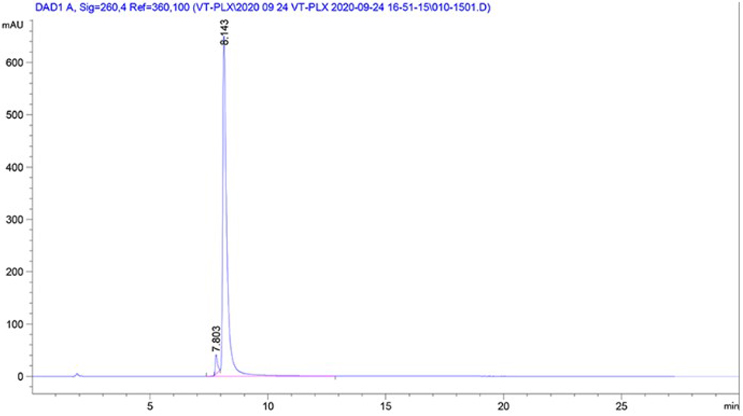
High purity plasmids with high supercoiled DNA and low endotoxin level
Our unique manufacturing process includes a patented soft DNA extraction process which simplifies the downstream purification to a single chromatographic step, leading to high purity plasmids with >90% supercoiled DNA. Endotoxins in purified plasmid DNA can decrease transfection efficiency and viability in cell lines and standard DNA preparation may therefore require additional endotoxin purification. We have optimized the plasmid manufacturing process to ensure the lowest endotoxin level possible in our plasmid preparation (<2EU/mg).
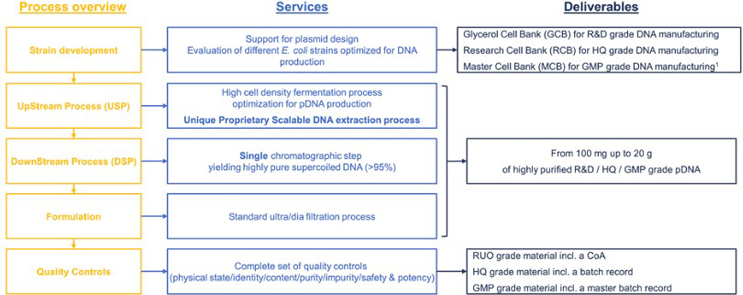
Unique scalable proprietary process from Research to GMP grade
Using the same process from Research to GMP grade no additional process optimization is required when scaling up or going for a higher quality grade. By using similar fermenters and the same proprietary and IP free E. coli strain, the same process can be used for manufacturing all quality grade plasmids (Research, HQ or GMP), whatever the type of plasmid (AAV, LV, mRNA, DNA vaccinez) hence saving time and cost.

From research to injectable GMP grade
Our different quality grade plasmids are provided with different deliverables.
Polyplus also offers E.coli cell banking services, including Glycerol, Research, and Master Cell Banks, and provide a Non-Animal Origin statement for all our products. Our injectable GMP grade plasmids are manufactured following the ICH Q7 guidelines and the Eudralex vol. 4 Part II standards, ensuring the highest safety for the patients. Our services include full-length sequence analysis using NGS or Sanger sequencing and a Certificate of Analysis for each batch of product. All our manufacturing and analytical batch records are supported with change control and environmental monitoring to ensure consistent quality. Polyplus has qualified equipment and a qualified workforce, and our GMP-inspected clean rooms meet ICHQ7 and Eudralex vol. 4 Part II standards. The produced plasmids undergo extensive characterization and quality control testing. Traceability of raw materials and processes, as well as batch to batch consistency are assessed to ensure robustness and reproducibility.
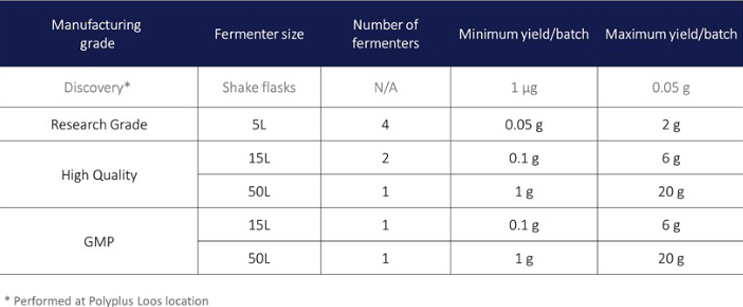
The manufacturing process uses different fermenter sizes in order to provide the desired quantities of plasmid DNA and therefore meet the needs for different applications.
