Pitfall Prevention: How Optomechanical Engineering Drives More Efficient Product Development
By Nick Smith and Jeff Treptau, Minnetronix Medical and Nichola Desnoyers and Mélanie Leclerc, INO

In optical device innovation, the ideal path from lab to launch is a straight line from Point A to Point B. Yet as many product teams can attest, unexpected pitfalls can add detours and delays along the way. Streamlining the process, however, are optical and optomechanical experts who can help translate a concept to reality on the journey to scalable production and market adoption. Working together in close collaboration, their combined skills and expertise can help device development teams build for success.
Right From The Beginning: Early Decisions & Design Ideas
The conceptual stage opens the door for exploration and achievement. More and more medical innovations incorporate optics, and as soon as there’s a concept for a new product or product enhancement, it’s time to start incorporating the inputs of optical and optomechanical professionals in three interrelated areas.
- Materials
In today’s medical technology world, there have never been more choices in materials available for manufacturing — and not just for core raw materials such as aluminum, stainless steel, and titanium. What about the adhesives used for bonding optics to mounts? Or anodization options for efficient product sterilization? As early decisions are made, close collaboration among optical designers and optomechanical engineers is essential.
- Performance
During early-stage development, a series of decisions based on the specific application of a device must be made, largely involving how lenses are mounted. For higher performance, a team may select a centering machine solution. For lower precision, the choice might be a drop-in lens barrel design. Additional considerations might then include an active focus or the degrees of freedom needed for alignment. Costs, of course, are always factors in decision making.
- Environmental
It’s never too soon to think about where and how a device will be used and also what the manufacturing environment will be. Temperature, humidity, dust, vibration, and light sources must all be accounted for, particularly if an optical device is portable and exposed to adverse field conditions. Even during manufacturing, will a cleanroom be required? Environmental considerations are all-encompassing.
Gaining Speed, Dialing In Success: Design Transfer
On CAD, everything looks good. Great, in fact. The concept is taking shape, and the next series of steps can start locking in a successful product launch that’s on time and within budget. Here’s how optomechanical engineers can support the team at this critical phase.
- Flowdown
Once optical designers and optomechanical engineers have determined the steps for assembly and alignment, it’s on to initial testing. Here, the flowdown of verification proceeds one subassembly at a time, and streamlining the process can save countless steps later on. The earlier a subassembly can be deemed to conform, the better the outcomes for maximizing yields and minimizing rework.
- Degrees of Freedom
On a lab bench, almost anything is possible. Skilled, patient designers can take their time adjusting the mechanisms that align an optical device. Not so in the field or a clinical setting. Fewer adjustments are better. Achieving optimal performance with minimal effort is the goal. Fabrication errors can render the most sophisticated design useless.
What’s called for during the design transfer stage is an optical tolerance analysis to determine the control necessary for achieving consistent precision. This step starts by involving suppliers as early as possible because custom and specialized components may need unique precision tolerances that will affect the manufacturing process and subsequent testing and validation. Ultimately, degrees of freedom have to be orthogonal, when possible, to facilitate alignment, and the design team works through an iterative process to make final recommendations on useability.
- Design for Manufacturability
Before a product can go to market, it has to come off the manufacturing line. Decisions made in preparation for manufacturing can have far-reaching implications. Adhesives, for instance, may need to meet certain thermal requirements for environmental conditions, and cure times are important for manufacturing. Can a component be designed to be common across multiple system variations? Or can the component be designed to interface seamlessly with temporary manufacturing assembly or optical testing fixturing? Smart design for manufacturing calls for both optical and optomechanical expertise.
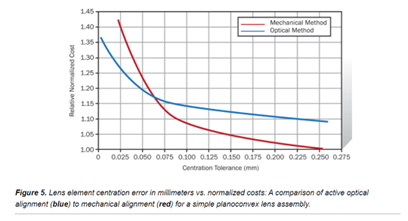
Developing an optimal product solution involves both optical and optomechanical expertise. Shown here, is an example demonstrating the cost-effectiveness of a lens assembly comparing active to mechanical alignments across a range of centering requirements.
Getting Better All The Time: Iteration & Continuous Improvement
Proactive collaboration brought the project from concept to near-reality, and continued team interaction with optomechanical input now can get it to the finish line. At this point, the complexities of the device are well-documented. How best to proceed with the goal of high-volume production and reliable clinical use? From an optomechanical perspective, testing at the subsystem level can help verify compliance of the technology stackup as a whole.
- Testing and Validation
Quality assurance (QA) during optical device manufacturing takes place as components are assembled and aligned, long before a product is ready to ship. As manufacturing scales to higher volumes, QA may involve testing each subassembly individually or one sample per unit. Optical designers and optomechanical engineers can help establish QA benchmarks because of the many earlier decisions they’ve made. They may, in fact, be able to suggest simulations, as a viable cost-saving option.
- Managing Revisions
Prototype by prototype and upgrade by upgrade, design solutions keep advancing. In medical device development, clinical validations are essential along with regulatory approvals. At every step, it’s important to track revisions for optical, mechanical, and electronic componentry. Optical designers and optomechanical engineers play a vital role in this process, and the history of changes—which includes all associated technical and system documentation—can further fuel further productive collaboration.
Clearly, the journey from a product idea to market-ready, scalable device requires informed decision making and calculated trade-offs throughout the development process. Optical and optomechanical experts can provide insight and expertise at every stage and prepare for next-generation advancements. From microns to millimeters, innovation is measured by aligning every detail for a complete and comprehensive solution.
About The Authors
Nick Smith is Senior Optomechanical Engineer for Minnetronix Medical; Jeff Treptau is Senior Optical Engineer for Minnetronix Medical; Nichola Desnoyers is Optical & Optomechanical Lead for INO; and Mélanie Leclerc is Optical Designer for INO.
Graphic courtesy of Trey Turner, Matt Lachapelle, and Roger Kirschner, Reo Inc., Active Alignment Techniques Improve Lens Centering, Photonics Spectra, December 2014.
