New Grafting Device Results in Improved Surgical Procedure

A revolutionary new grafting device from Guidant Corp. is providing abdominal aortic aneurysm (AAA) patients with a surgical alternative.
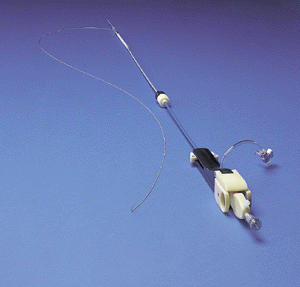
Both Guidant's Ancure Endograft system and traditional surgery aim to repair an aorta that has been weakened by an aneurysm, a bulge brought on by damage to the blood vessel. An untreated aneurysm can burst under the pressure of flowing blood, often causing death. Both methods allow blood to flow freely through the aorta without the risk of aneurysm rupture.
The surgical method of treating AAA – in which a surgeon enters the body through an incision between the breast and pubic bones, opens the aneurysm and sews a graft into place – is sometimes more dangerous for the patient than the condition itself. The surgery requires a lengthy recovery period.
How the Device Works
Using the Ancure Endograft system, surgeons cut two small incisions in a patient's groin area, and, using an x-ray imaging device and catheter delivery system, gently guide the one-piece polyester graft through the femoral artery in the upper leg and into the aorta. A four-step deployment system allows the surgeon to release the graft inside the aneurysm itself, where it secures itself to the aorta with small metal hooks that act as sutures.
Once discharged, most patients find themselves back to their normal activity level by two weeks. It usually takes six to eight weeks after traditional open surgery.
Finding the Right Material
When planning the Ancure delivery catheter, Guidant turned to Bayer Corp.'s Plastics Division to develop a handle that would respond to surgeons during the procedure. All components of the handle are made of Bayer plastics – Makrolon polycarbonate and Lustran acrylonitrile-butadiene-styrene (ABS) resins.
"Bayer offered a number of advantages to this project," said John Ordway, purchasing manager at Guidant's Cardiac and Vascular Surgery Group. "The catheter handle is used to guide the graft into the patient's body – it had to be designed for surgical performance. The Lustran and Makrolon materials allowed us to develop a handle for the catheter that is comfortable, responsive and easy for surgeons to use."
According to Ordway, the Ancure Endograft system was approved for use in late 1999 and was highly anticipated by surgeons and patients alike, some of whom even postponed surgery until the device was ready. Bayer played a role in speeding the development process for Guidant.
"Bayer's medical grade plastics helped Guidant save valuable time and money that otherwise would have been spent on lengthy and expensive testing procedures," Ordway said. "Other companies have similar products, but Bayer is more supportive of our needs; we get the plastics we want when we want them – not later. Bayer is simply committed to the medical market."
For information on Guidant's Ancure Endograft system, contact: Guidant Cardiac and Vascular Surgery Group, 1525 O'Brien Drive, Menlo Park, CA 94025.
For information on Makrolon polycarbonate and Lustran ABS resins, contact: Bayer Corp., Polymers and Chemicals Communications Group, 100 Bayer Road, Pittsburgh, PA 15205. Tel: 1-800-622-6004.
Source: Medicaldesignonline.com, sister Website to Nurses.com
