First-Time Imagery Of Tumor-Related Enzyme Reveals New Insights
By Chuck Seegert, Ph.D.
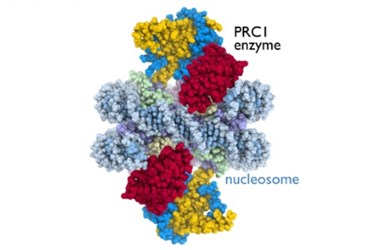
For the first time, Polycomb Repressive Complex 1 (PRC1) was imaged as it interacted with cellular machinery. Crystallographic images of the cancer-related enzyme may give insight into how proteins regulate genetic activity in disease processes.
The structure and anatomy of normal tissue is tightly controlled by the genetic and cellular machinery that makes up the tissue. During cancer development, certain cell processes aren’t turned off when they should be, and cell growth is no longer controlled. This allows tumor cells to accumulate in ways they normally wouldn’t.
An important enzyme that activates and deactivates cellular activity is the PRC1 protein, according to a recent press release from Penn State University. This enzyme interacts with another cellular structure called a nucleosome and, when they interact, they control certain genetic activities.
"The nucleosome is a key target of the enzymes that conduct genetic processes critical for life," said Song Tan, professor of biochemistry and molecular biology at Penn State University and the leader of the study's research team, in the press release.
While the interaction between PRC1 and nucleosomes was known to happen, it historically was unclear as to how the two structures came together, according to a study published by the team in Nature. By growing crystals of the protein and nucleosome complex, the team was able to use X-ray crystallography to form a clear molecular picture of the binding sites between the molecules.
Interestingly, the interaction between PRC1 enzymes and nucleosomes may have implications with other cancer-related proteins — like the BRCA1 breast cancer-associated tumor-suppressor — and could help develop a better understanding of that disease process, according to the press release.
“Our study suggests that BRCA1 and PRC1 employ a similar mechanism to anchor to the nucleosome,” Tan said in the press release.
X-ray crystallography is a mainstay of molecular biology research, but it is a laborious process that takes tremendous amounts of time to perform. New analysis methods, however, have simplified this process and may allow access to many more researchers, according to a recent article on Med Device Online.
Image Credit: Credit: Song Tan lab, Penn State University
