Nanowires For Greener LEDs
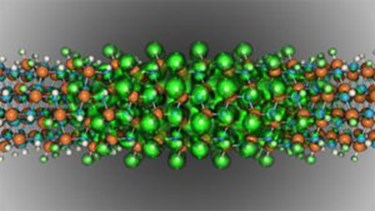
Nanostructures half the breadth of a DNA strand could improve the efficiency of light emitting diodes (LEDs) right where they need it, Michigan Engineers have shown. At present, efficiency plunges for green light, but semiconductor nanowires could change that.
“Our work suggests that indium nitride at the few-nanometer size range offers a promising approach to engineering efficient, visible light emission,” said Emmanouil Kioupakis, an assistant professor of materials science and engineering.
LEDs are semiconductor devices that emit light when an electrical current is applied. At low power, nitride-based LEDs (most commonly used in white lighting) are very efficient, converting most of their energy into light. But turn the power up to levels that could light up a room and efficiency plummets, meaning a smaller fraction of electricity gets converted to light. This effect is especially pronounced in green LEDs.
Better green LEDs could make direct red-green-blue color mixing practical in ambient lights. This would allow engineers to fine-tune the light to pleasing hues, or develop a way to control the warmth or coolness of the light according to the task or time of day. Instead, most white lighting today comes from blue LED light passed through a phosphor, a solution similar to fluorescent lighting and not a lot more efficient.
While the semiconductor indium nitride usually emits infrared light, Kioupakis and his PhD student Dylan Bayerl found that size matters. Using a supercomputer at the National Energy Research Scientific Computing Center, they simulated how the semiconductor would behave if grown in wires just one nanometer (0.000001 millimeters) across. They found that wires could glow green much more efficiently than current LEDs.
The shift from infrared to green light emission occurs because the thinness of the wires creates a different landscape for the electrons. Light emission starts with an electron taking some electrical energy and jumping up to a higher energy state. When the electron jumps back to its original state, the energy is released as light.
In the narrow confines of the wires, it takes more energy for the electron to jump from one state to another, which in turn means more energy goes into the emitted light. The extra energy moves the light from the infrared spectrum to visible colors.
“If we can get green light by squeezing the electrons in this wire down to a nanometer, then we can get other colors by tailoring the width of the wire,” said Kioupakis. A wider wire should yield yellow, orange or red – a narrower wire, indigo or violet.
The researchers believe that pure indium nitride nanowires improve efficiency in three ways. Semiconductor alloys in typical microchip LEDs contain variations in composition, and this takes a toll on efficiency. So does the mismatch in atom spacing between layers of different materials. Since the nanowires are all one material, they don’t have either of these problems.
Finally, the reduction in space may help more electrons succeed in emitting light. When the electron jumps from one level to another, it leaves behind a positively charged “hole.” If that hole is physically closer to the electron due to the tiny dimensions of the nanowire, it’s more likely that the electron will be pulled back to the hole and emit light rather than releasing the energy as heat.
“Bringing the electrons and holes closer together in the nanostructure increases their mutual attraction and increases the probability that they will recombine and emit light.” Kioupakis said.
Other improvements over microchip LEDs include thinness, better flexibility and higher resolution.
While this result points the way toward a promising avenue of exploration, the researchers emphasize that such small nanowires are difficult to make. However, they suspect their findings can be generalized to other types of nanostructures, such as embedded indium nitride nanocrystals, which have already been successfully produced in the few-nanometers range.
Their results, published online in February as “Visible-Wavelength Polarized Light Emission with Small-Diameter InN Nanowires,” will be featured on the cover of the July issue of Nano Letters.
The simulations ran at the U.S. Department of Energy's National Energy Research Scientific Computing Center (NERSC), located at Lawrence Berkeley National Laboratory. Burlen Loring of NERSC’s Analytics Group created visualizations for the study, including the journal’s cover image. The researchers also used the open-source BerkeleyGW code, developed by NERSC’s Jack Deslippe.
This work was supported as part of the Center for Solar and Thermal Energy Conversion, an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science.
Source: Lawrence Berkeley National Laboratory
