From "Guess" To "Best": How Infrared Cameras Are Increasing Accuracy And Insight In Product Testing
By Chris Bainter, Business Development Manager, R&D/Science Solutions, FLIR Systems, Inc.
Infrared (IR) cameras may be best known for their use in military and security applications. Yet the same attributes that enable IR cameras to thrive in these environments – namely their ability to detect infrared radiation and form an image with it – make them ideal tools for product testing and product development in the medical devices field.
Once thought too expensive for common use in the product testing process, today's IR cameras are more affordable than ever. They serve as a strategic testing tool that speeds product development by providing major data-gathering advantages over legacy approaches to temperature measurement. These advantages can lead to insights critical to preventing costly product failures and safety hazards, providing operations that use them with an immediate and ongoing return on investment.
As medical device manufacturers develop new products, the key temperature measures they must understand, track, and manage include: understanding the ways in which heat impacts medical device performance, identifying hot spots and temperature spikes, and knowing how, and the rate at which, materials in a device heat up and cool down. Yet even as the rate of innovation in the medical device field has increased, temperature measurement approaches have remained relatively unchanged.
Two Types of Temperature Measurement, One Common Problem
Two types of temperature measurement tools are prevalent today in medical device testing: probes and pyrometers. First, let's consider probes, which include thermocouples, resistance temperature detectors (RTDs), and thermistors. The problem with probes is they require direct contact with the surface of the device being measured and they only produce a single measurement – the temperature of the specific spot where that contact is made.
There are several disadvantages to this "single point of contact" approach:
- A single point of measurement is, well, limiting. If you happen to have the prescience to know the exact point that needs measuring, this approach may prove sufficient. It's not a good approach, however, for finding a "hot spot" – that potentially dangerous area where your device might overheat and cause performance issues or harm. The workaround that product testers typically employ here is to either blanket their device in probes in order to deliver multiple temperature measurements, or move a single probe over the device to achieve a similar effect.
The problem, of course, is that neither of these approaches is particularly practical, nor accurate. Nor do these approaches lend themselves to accurately characterizing thermal gradients, or the rate of temperature change over a defined area on a device or object. Similarly, you can't use these tools to find the maximum or minimum temperature or peak temperatures across the device. Simply put, there are many dimensions to how heat affects device performance: measurement approaches that deliver one single point of temperature data at a time don't fully reveal behavior that can be indicative of points of failure, trouble, or concern.
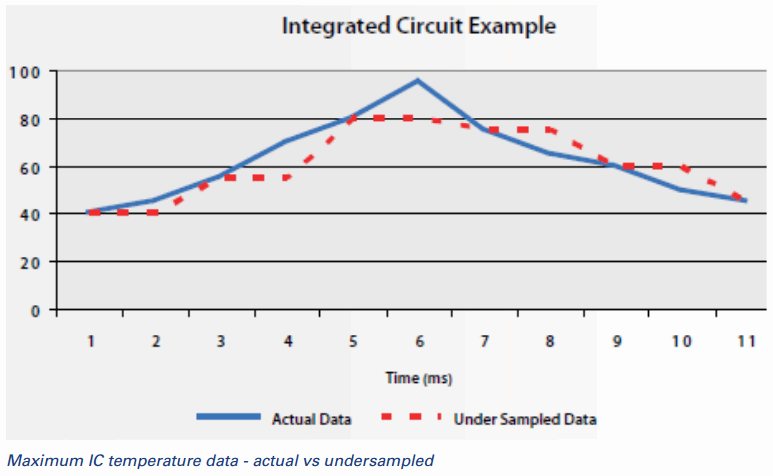
- "Single point of contact" measurement approaches are also constrained by the frequency with which they can deliver data. Put another way, they're too slow. Medical devices such as surgical cutting and cauterizing tools can heat up quickly, displaying a fast thermal transient. Measuring this rapid heating accurately in the design phase requires rapid temperature sampling and measurement. If you are using a single probe to make this measurement, you are missing something. You might miss the true maximum temperature to which the device has heated, or a temperature spike during the heating process. What's worse, you won't even know what you have missed, which effectively means you are basing product design and performance decisions on bad data. When you don't sample fast enough, you don't know what you don't know.
![]()
- The physical challenge of mounting a probe to a medical device is daunting. Yes, it can be done, but did you know that getting an accurate measurement actually requires cementing the probe to the device?We know from experience that product testers often prefer to either rest a probe on a device or tape it in place, but these practices can yield wildly inaccurate data (FLIR has an entire paper dedicated to this topic). Then, think about the challenges involved when the surface of the object to be measured is physically smaller than the probe itself, or when the surface to be measured is in motion, such as a fan blade or an air bag. In each of these instances, mounting a probe is essentially a non-starter.
 Some medical device manufacturers have attempted to move past these problems by deploying the second type of temperature measurement tool: a spot pyrometer. Pyrometers don't require physical contact with the object being measured, but these measurement devices have their limits and liabilities.
Some medical device manufacturers have attempted to move past these problems by deploying the second type of temperature measurement tool: a spot pyrometer. Pyrometers don't require physical contact with the object being measured, but these measurement devices have their limits and liabilities.
Spot pyrometers, like thermocouples and other probes, can only deliver a single point of temperature measurement. They may seem a smart, economical alternative to IR cameras in that they don't rely on physical contact with the object to measure it, but they fall short on the data they deliver. This is because using a spot pyrometer is a bit like shining a flashlight at a wall: the circle of light grows larger and less distinct as you move away from the wall. When the spot pyrometer is close to an object, the device measures a smaller area, but as the device is moved farther away, the measurement area increases. Instead of a single point, the temperature reading represents an average of the entire circle. If your hot spot is contained within that larger area, the measurement you get for it won't be accurate.
The Dangers of Settling for "Good Enough"
Medical device makers need a better way than these legacy approaches to measure temperatures in order to understand with precision where their devices are susceptible to overheating, breaking down, and performing out of specification. Without accurate measurement data, the following problems can ensue:
- Missing potential points of failure. Not having enough data, or, worse, having inaccurate data, can blind a manufacturer to possible points of product failure. The consequences of this are all too familiar: either the product doesn't last as long as it should, or it doesn't perform reliably within specification, leading to product failure and safety issues. For example, consider the aforementioned cutting and cauterizing device. If we don't sample fast enough or miss a temperature spike during testing, the device could scorch the tissue. Another example is a temperature spike on an electronics component during operation that is overlooked, which is "wounding" the device. This may result in a device that's expected to last for five years but will now fail after only three.
- Overdesigning products. Medical device makers could fall into the trap of overdesigning products when attempting to avoid temperature hazards. While patient safety is paramount, countering bad data by overbuilding the product can result in devices that are larger, heavier, and require more power. They could also be more costly, making them less desirable than the competition. Instead, accurate measurement can create a win-win for manufacturers and patients alike: increased product safety at a lower price.
- Repeating mistakes. Everyone involved in making, selling, and servicing a product knows the perils of using bad data. When inaccurate or incomplete temperature data from the product testing process is fed into a company's product models and simulations, a host of bad downstream consequences can occur – and reoccur.
The IR Advantage
If you recognize any of your company's current testing and measurement processes in these prior descriptions, take heart. There is a better way. Infrared cameras are no longer a luxury item reserved for limited use. They are, in fact, ideally suited to the rigors of medical device testing and can put your team on the fast track to developing better, safer products more quickly and reliably. Here's how IR cameras address the shortfalls of legacy measurement instruments:
- More data, no contact required. Depending on the resolution of the model you are using, IR cameras can deliver millions of points of non-contact temperature measurement at the push of a button. Put another way, you will get a temperature measurement for every pixel within every image taken, enabling you to thermally characterize your device "spatially" (i.e., across the device) with accuracy.
- Faster measurement. IR cameras are much faster than legacy measurement tools, so you can also characterize your device "temporally." The ability to accurately characterize thermal transients over time, through the speed of the IR camera, is particularly important in medical devices such as dental drills or those that use laser pulses. These fast-moving tools heat quickly and require an equally agile measurement approach to capture their thermal behaviors.
Note: Gaining thermal accuracy across these two dimensions (time and space) helps product testing in different ways. First, thermal imaging helps testers doing a failure analysis identify hot or cold spots or unusual temperature gradients in different areas. This enables developers to troubleshoot or identify potential points of failure or concern. Second, being able to see and accurately characterize temperature spikes in time and space helps developers ensure the product is operating within spec and then begin fine-tuning the product to perform even better within that realm. Third, gaining this wealth of non-contact data is helpful when evaluating a returned product, giving the team a quicker way to find the fault and where to focus further investigation.
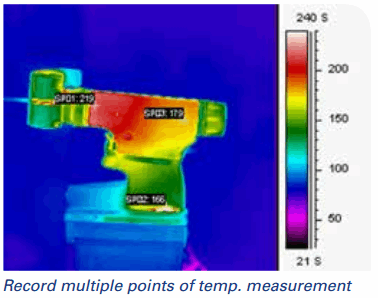
- Measuring the smallest areas accurately. IR cameras help test the smallest devices, surface areas, and electronics. When the area you want to measure is smaller than a probe, IR cameras are the answer. When companies use thermocouples to measure small areas, they effectively create a heat sink that corrupts the data. With a spot pyrometer, if the object that needs to be measured is smaller than the pyrometer's "beam," you won't get an accurate temperature. With an IR camera, you can use optics to get down to less than a 5 micron per pixel spot size. Again, since these cameras can measure temperature for every single pixel, they can actually provide accurate readings on much smaller targets than traditional forms of temperature measurement.
- Great data, rapid prototypes. Faster, more accurate data can dramatically improve a product developer's simulation and modeling programs, creating its own virtuous cycle. Such programs, informed by better, more accurate, real-world data, now shorten the product development cycle. Instead of having to build a new prototype every time, companies can run simulations that are informed by the best data. This speeds product to market through rapid prototyping.


Conclusion
How many man-hours does the development of a medical device product take? Thousands, tens of thousands, even more? While every company is looking for ways to streamline this process without adversely affecting product quality, using outdated or mismatched tools in product testing isn't the answer. IR cameras are ready now to play a prime time role in improving product testing and development by delivering accurate temperature measurement results quickly and accurately across device types and in ways that, by degrees, make each product and process perform better.
Click here to download the PDF version.