Optical Microscopy Takes A Hyperspectral Stroll To The Dark Side
Multimodal spectral imaging now possible using any optical microscope
A team of scientists at Pacific Northwest National Laboratory (PNNL) have demonstrated how any research-grade optical microscope could be used to record spectrally resolved optical images. Their initial demonstration focused on recording dark field spectral images of isolated silver nanospheres.
The team essentially replaced a standard two-dimensional (2D) camera of an optical microscope with a 3D "hyperspectral" detector. The resulting instrument was then used to record spatially (2D) and spectrally (adding a dimension) resolved dark field optical images of hundreds of silver nanoparticles in a matter of ~30 seconds.
"Hyperspectral dark field optical microscopy is just the beginning. Absorption and potentially fluorescence-based spectral imaging of a variety of target systems is a natural extension of this work," said Dr. Patrick El-Khoury, lead author of a paper recently published in Journal of Physical Chemistry C. These target systems include live cells, biological specimens, engineered metallic substrates with unique optical properties, and atmospheric nanoparticles.
Why It Matters: The role of optical microscopy across all branches of science is undisputed. To be able to "see" images of objects that could otherwise not been seen by the naked eye has led to huge advances in the life and physical sciences.
The information content in standard optical microscopy images is limited. "Seeing" is one thing; chemically identifying the microscopic samples is an entirely different story.
To chemically identify microscopic specimens would require interrogating, for example, the excited electronic and/or vibrational states of a material system. The latter is the realm of optical spectroscopy, which, combined with microscopy, has led to significant advances in the life and physical sciences.
In general, hyperspectral optical imaging involves transforming 2D (spatial) images into 3D images (spatial and spectral). This requires specialized instrumentation that image and identify (bio)material systems in 3D through their vibrational or even their electronic states. The PNNL team's approach simplifies the general problem. It relies on the combination of a readily portable hyperspectral imager, about the size of an artisanal loaf of bread, which can be easily coupled to any research-grade optical microscope with a standard camera mount.
Because this approach requires neither a scanning microscope stage nor software enabling stage-detector communication, the detector can be used across multiple microscopy platforms. These could be bright field, dark field, or fluorescence microscopes.
The simplest of all optical microscopy visualization techniques is bright field microscopy, whereby light is transmitted through a sample, and its loss in intensity causes contrast across a specimen's image. In dark field optical microscopy, the directly transmitted light is blocked; only the photons that are "scattered" by the specimen are detected. A dark background in the absence of a scattering specimen is characteristic of dark field optical images, whereby the attainable contrast is significantly enhanced.
Standard cameras record real-color images of a sample under bright or dark field illumination in a microscope. The best spatial resolution attainable is about 200 nanometers (nm), or 200 billionths of a meter, when visible light is used to illuminate a sample. To give perspective, the typical resolution is small compared to most biological cells (1-100 micrometers, or µm); however, it is huge compared to viruses (100 nm), proteins (10 nm), and isolated molecules (1-2 nm).
Methods: To provide context for the approach, El-Khoury gave this macroscopic example: "If you were to snap a picture of a blue wall with a red door, you would be able to infer the colors in the scene from the image. With this technology, you can essentially reconstruct the scene, one color at a time. In other words, one ‘slice' would comprise nothing but the wall, whereas only the door would be visible in another slice. Now think of doing this in a microscope, all while replacing ‘color' with more chemically/analytically meaningful quantities."
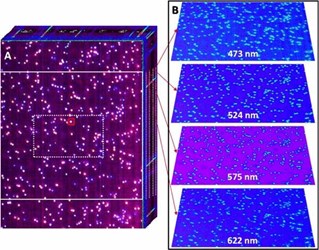
Using dark field illumination in an optical microscope equipped with the team's hyperspectral imager, they recorded 3D dark field optical micrographs of hundreds of silver nanoparticles (100 nm in diameter), as shown in the figure above. The 3D image cube consists of a stack of dark field optical images. In other words, each pixel contains spectral information in the visible region of the electromagnetic spectrum.
Plasmons are collective charge density fluctuations at the surface of the metallic structures, a phenomenon having numerous applications in fields as diverse as ultrasensitive chemical detection and nanoscale chemical imaging, targeted drug delivery and therapeutics, and photovoltaics and photocatalysis, to name a few. Besides their general interest in plasmonics, the PNNL team opted to record spectrally resolved dark field scattering from isolated plasmonic nanoparticles for two main reasons. First, the ability to record the spectral images of single plasmonic nanoparticles significantly advances ongoing quests aimed at understanding the vibrational signatures of single molecules in proximity to plasmonic nanostructures. The second reason has to do with the proof-of-principle nature of the study. Light scattering is a relatively inefficient process. As such, demonstrating that hyperspectral dark field optical micrographs of single particles can be recorded indicates that other imaging modes, such as hyperspectral optical absorption microscopy, are feasible using the same instrument.
The sample consisted of a sparse distribution of 100-nm silver nanoparticles on a glass coverslip. Three-dimensional image cubes were recorded by projecting a narrow line of an optical micrograph onto a 30-mm-wide slit, dispersing the slit image through a grating onto a 2D sensor, and scanning the slit across the field of view dictated by the microscope objective to span the second (y-) spatial dimension. The hyperspectral detector was equipped with an internal slit scanner. As such, 3D image cubes were obtained without the need for sample stage scanning.
The spectral coverage of the imager spans the 377- to 1051-nm spectral region at a spectral resolution of ~5 nm. Hyperspectral image cubes were recorded at a rate of 25 seconds per cube, all while retaining ample sensitivity. The recorded images were spatially calibrated using National Institute of Standards and Technology-traceable standards. Using a 100X microscope objective with a numerical aperture of 0.75, diffraction-limited hyperspectral images were sampled at ~84 nm2/pixel over a field of view of 43.7 µm x 58.5 µm.
What's Next: Several future directions are envisioned by the PNNL team. Whereas hyperspectral absorption microscopy of biological samples is already under way, extending the spectral coverage of the detector and adding another spatial dimension for a total of four dimensions (x,y,z,l) are two natural extensions to this work. The team is also open to being challenged by new and different types of samples to test the versatility of their setup and explore its limits.
Source: Pacific Northwest National Laboratory
