Enhanced Protein Fluorescence Yields Better Neuronal Observations
By Chuck Seegert, Ph.D.
Studying neural activity is a delicate process that historically has been done using microelectrodes. While observing whole networks of neurons in a living organism has proven elusive, a new method developed by researchers from the California Institute of Technology (CalTech) may make this type of work possible.
For decades researchers have been trying to understand brain function at an organ level, while correlating it with behavior. Positron emission tomography (PET) does this on a certain level, but its resolution is limited. One goal is to be able to study impulses that travel along the neural cells as they happen to see how cells interact with others in their network. These impulses are called action potentials, which are small electrical variations that occur when the ions in a cell undergo a state of flux from outside to inside the cell. Usually this behavior comes from some sort of stimulus, like a scent or a touch.
"Our overarching goal for this tool was to achieve sensing of neuronal activity with light rather than traditional electrophysiology, but this goal had a few prerequisites," said Viviana Gradinaru, a professor of biology and biological engineering at CalTech, in a recent press release. "The sensor had to be fast, since action potentials happen in just milliseconds. Also, the sensor had to be very bright so that the signal could be detected with existing microscopy setups. And you need to be able to simultaneously study the multiple neurons that make up a neural network."
The first step in achieving this goal was to develop a special light-sensitive opsin, or light-sensitive protein called Archaerhodopsin (Arch). Details of how they optimized this protein were published in a study in the Proceedings of the National Academy of Science.
While the use of opsins is not new, the special requirements of the neural system required them to fluoresce much more brightly than previous versions. Using directed evolution, the researchers developed E. Coli strains, which enabled them to genetically optimize Arch by choosing the cells that created the brightest proteins over several generations. This refined the proteins made by the cells until they were about 20 times brighter than the original proteins.
"This experiment demonstrates how rapidly these remarkable bacterial proteins can evolve in response to new demands,” said Francis Arnold, the Dick and Barbara Dickinson Professor of Chemical Engineering, Bioengineering, and Biochemistry, in the press release. “But even more exciting is what they can do in neurons, as Viviana discovered"
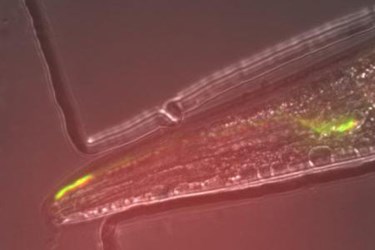
The next step was to take this protein and incorporate it into a living organism, which the researchers did using the nematode worm C. elegans, according to the press release. Using the worm’s olfactory system, they were able to show that when a scent was present, the circuit of neurons would light up. For the first time, an Arch variant was used to observe an active circuit in a living organism.
The field of optogenetics, which uses light to control and study neurons, is rapidly coming to the fore in neural science. Recently in an article published on Med Device Online, another opsin was shown to shut down neural activity when it was exposed to light.
Image Credit: Hui Chiu and Viviana Gradinaru/Caltech
